Hydrazine Formula - Hydrazine Uses, Properties, Structure and Formula
Hydrazine is an inorganic base which is an important reagent in the preparation of many nitrogen compounds.
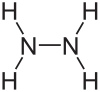
Formula and structure: The chemical formula of hydrazine is NH2NH2. Its molecular formula is N2H4, and its molar mass is 32.04 g/mol. The chemical structure is shown below, consisting of two NH2 groups covalently attached. Each of the N-NH2 groups adopts a pyramidal shape.
Occurrence: Hydrazine is produced naturally by some microorganisms such as yeast, bacteria and fungi, as it is an intermediate in the anaerobic oxidation of ammonia.
Preparation: The commercial production of hydrazine is by the Raschig process, in which sodium hypochlorite solution is treated with excess ammonia to form a chloramine intermediate, which then gives the final hydrazine product along with hydrochloric acid.
NaOCl + NH3 → H2N-NH2 + HCl
It can also be prepared in a related process by using urea (H2N-CO-NH2) instead of ammonia:
H2N-CO-NH2 + NaOCl + 2 NaOH → N2H4 + H2O + NaCl + Na2CO3
Physical properties: Hydrazine is a colorless and dense liquid with a strong odor of ammonia. It has a density of 1.02 g/mL and a boiling point of 114 °C. It is highly flammable and soluble in water.
Chemical properties: Hydrazine is a highly reactive base and reducing agent, and is widely used in organic synthesis. Hydrazine is a moderate base, while its aqueous solutions are highly alkaline. It reacts violently with oxidants, acids, metals and metal oxides, creating a potential fire and explosion hazard. When heated to decomposition, it emits toxic fumes of nitrogen oxide, ammonia and hydrogen, which can also lead to fires and explosions.
Uses: Hydrazine is used for many industrial applications including preparation of polymer foams, polymerization catalysts, pesticides and the gas used in air bags. Several important pharmaceuticals are based on hydrazine and its derivatives. Hydrazine is also used in various rocket fuels, in power plants, in organic synthesis and in fuel cells as a safer alternative to hydrogen.
Health effects/safety hazards: Hydrazine is a highly toxic compound despite its pharmaceutical applications. Exposure to hydrazine at high concentrations can cause irritation of the eyes, nose and throat, and also affect the liver, kidneys, and central nervous system. Severe exposure symptoms include headache, nausea, pulmonary edema, seizures, and even coma. Hydrazine solutions are corrosive and can cause skin burns.
|
Related Links: |
